Engineering Thermoplastics Guide. Chapter 1: Properties and Classification of Engineering Plastic Products
This chapter excerpt introduced an overview of the properties of different classes of thermoplastic materials, with special attention to engineering and advanced thermoplastics.
This is an excerpt from Chapter 1 of The Engineering Thermoplastics Guide. Download the full guide below for the full text, including technical diagrams and application notes.

Introduction
Plastic products are ubiquitous in our lives. Total plastics production is estimated to be over 50 Mtons in Europe alone, and almost 300 Mtons in the entire world. [1]
Based on their functional characteristics and uses, thermoplastic materials can be sorted into three main categories:
· Standard or commodity thermoplastics, such as HDPE and PP. They are relatively low-cost, high-volume consumption materials, mainly used for packaging and consumer goods. They constitute the largest portion of thermoplastic materials produced today, with a global consumption of about 30 times that of engineering plastics and 600 times that of the advanced plastics group.
· General-purpose engineering thermoplastics, such as PC and PA. These materials generally exhibit higher thermal and mechanical resistance compared to commodity plastics. They are used as durable components in a large number of engineering applications.
· Advanced thermoplastics, such as PEEK and PPS. These materials are suitable for applications under extreme thermal, mechanical, and chemical conditions, where they can substitute thermosets and metals. They can typically withstand temperatures of over 200 °C and possess high strength, stiffness, and toughness.
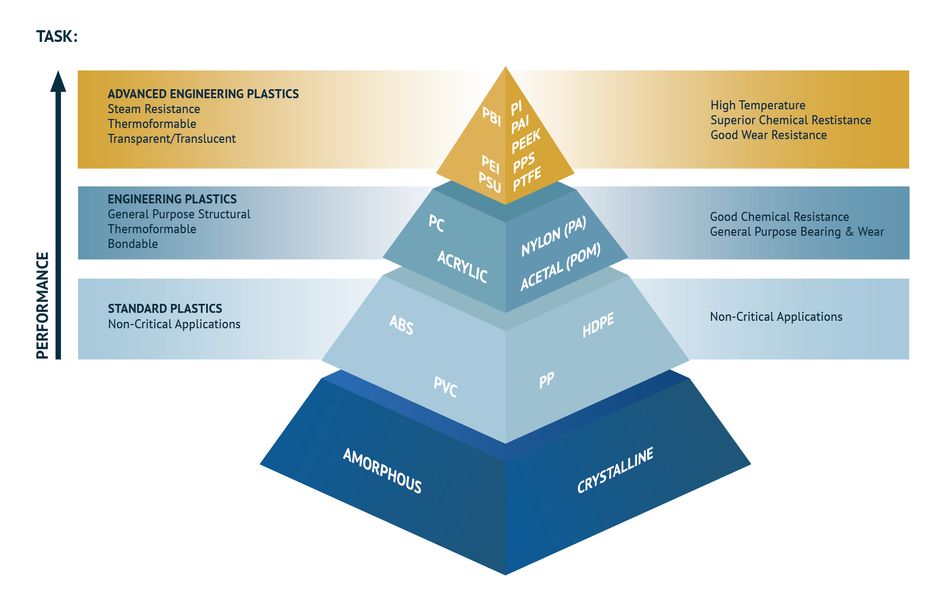
General Properties of Engineering Thermoplastics
Chain flexibility and mobility
In plastics, chemical microstructure is strongly tied to the material’s macroscopic properties. The structure-property relationship of thermoplastics is complex, but generally speaking, it boils down to chain flexibility, i.e. the freedom of movement of the atoms inside each polymer chain, and chain mobility, i.e. the freedom of movement of polymer chains with respect to each other.
Intrinsic chain flexibility is related to the energy required by molecules to rotate around chemical bonds. This, in turn, depends on the chemical structure of each polymer. If the polymer chain is linear and composed of mostly single aliphatic bonds, such as in the case of polyethylene (PE), polymer chains will be flexible. On the other hand, if the molecular structure contains aromatic groups, such as in the case of polycarbonates (PC) or polyether ketones (PEEK), the polymer chains will be more rigid.
Chain mobility between polymer chains is affected by molecular weight and chemical composition. If the molecules have high molecular weight or contain highly polar chemical groups capable of forming hydrogen bonds, chain mobility will be restricted.
Glass transition temperature and heat deflection temperature
The differences in chain flexibility and mobility are mirrored in the macroscopic properties of thermoplastics.[1] Glass transition temperature, or Tg, is defined as the temperature below which a plastic material behaves as a glassy solid. Smaller flexibility and mobility of the polymer chains lead to higher Tg. All engineering and advanced thermoplastics are high-Tg materials. This makes them more suitable for demanding applications due to their higher thermal and mechanical resistance.
In engineering, another significant parameter to understand the thermal properties of thermoplastics is heat deflection temperature or HDT. This parameter is a measure of the effect of temperature on the resistance to distortion of the thermoplastic under load. In other words, if a thermoplastic material has a high HDT, it is more likely to preserve its shape and mechanical properties even at high temperatures.
Crystallinity
Thermoplastics are classified as either semi-crystalline or amorphous. Put simply, crystallinity is a measure of the degree of order in the arrangement of the polymer chains. While amorphous thermoplastics have a random molecular arrangement, semi-crystalline thermoplastics possess a regular molecular structure.[1]
This has significant consequences on the functional properties of plastic products. Semi-crystalline thermoplastics, such as polyethylene terephthalate (PET) or PEEK, typically possess higher mechanical strength and stiffness compared to amorphous materials. They also tend to exhibit better chemical resistance.
Conversely, amorphous thermoplastics, such as polysulfone (PSU) or polyetherimide (PEI), are flexible and possess higher impact resistance. Because they tend to be transparent, they are also employed for optical applications.
Classification of Thermoplastic Products
Advanced Engineering Plastics
Advanced engineering plastics possess high mechanical strength and stiffness even at extreme temperatures, between 120 °C and 230 °C. Some advanced thermoplastics can now be used at temperatures surpassing 400 °C. These materials also tend to possess broad chemical stability and excellent electrical properties. They are suitable for the most challenging and demanding applications.
Imidized polymers (PEI, PAI, PI)
The family of imidized polymers is characterized by the presence of at least one imide chemical bond (CO-N-CO). This group includes thermoplastic imides (PI), polyamide-imides (PAI),polyetherimides (PEI), and polybenzimidazole (PBI).
Pure polyimide (PI) can withstand temperatures up to 360 °C, which makes it suitable for demanding applications as a lightweight alternative to metal. It offers high strength, toughness, and chemical resistance. However, its high Tg means it is not easily melt processable. [1]
PEI was first developed to overcome this processability issue. This amorphous thermoplastic with high HDT, high tensile strength, and modulus ensures a reliable performance at high temperatures. Additionally, PEI’s electric properties remain stable over a wide range of frequencies. It can undergo repeated autoclave treatments. It is also flame-retardant and is resistant to hydrocarbons, alcohols, and acids. [2]
PAI’s flexible amide bond makes processability easier while retaining the outstanding thermal and chemical resistance properties of PI. This, combined with its optimal dielectric properties, makes it one of the most suitable materials for demanding applications in electronics.
All imidized polymers are hygroscopic, meaning they tend to absorb moisture. Therefore, parts must be carefully dried before use to prevent damage and ensure optimal performance. [3]
Sulfone polymers (PSU)
Polysulfone (PSU) is an amorphous translucent thermoplastic. Chemically, it is characterized by diphenyl sulfone units.
Structure of PSU
Its chemical structure offers excellent stability, resulting in a broad temperature range, excellent mechanical properties, and good short-term chemical resistance to solvents and weak acids. It is lightweight and relatively inexpensive. It possesses good dimensional stability and generally is resistant to beta, gamma, and X-ray radiation. [1]
Polyphenylene sulfide (PPS)
Polyphenylene sulfide (PPS) possesses a regular, semicrystalline structure.
It exhibits the lowest moisture absorption of all advanced thermoplastics and is inherently flame-retardant. It possesses outstanding chemical resistance to both corrosive substances and solvents even at high temperatures. The low coefficient of thermal expansion provides optimal dimensional stability. Its mechanical properties depend on the degree of crystallinity: although the material has a high E-modulus, highly crystalline PPS can be relatively brittle. [1]
Polyether ether ketone (PEEK)
PEEK is a semi-crystalline thermoplastic with outstanding thermal and mechanical properties. Similarly to other advanced thermoplastics, it owes its properties to its peculiar chemical structure, containing phenyl and ketone groups which offer high stability and rigidity.
PEEK possesses a high E-modulus and tensile strength. It melts at 350°C and is resistant to high temperatures. Its chemical resistance to organic solvents is also outstanding, and it is not hydrolyzed by either water or high-pressure steam. Very good resistance to radiation is another feature of this advanced plastic material. [1]
Polybenzimidazole (PBI)
Polybenzimidazole (PBI) is an amorphous thermoplastic. It can be classified as an extreme thermoplastic material, exhibiting the highest thermal stability of all advanced thermoplastics. It can withstand temperatures as high as 430°C for prolonged periods, and above 500 °C for up to a few hours.
Above 200°C, high molar mass PBI possesses the highest mechanical properties than any other unfilled plastic material. It does not burn and preserves its mechanical characteristics even when charred. Because of this, it is one of the most outstanding advanced thermoplastic products available on the market. [1]
Fluoropolymers (PTFE)
Fluoropolymers, such as PTFE, are characterized by the presence of highly stable carbon-fluorine chemical bonds.
This chemical stability, coupled with high crystallinity, makes PTFE especially heat-resistant, even at high temperatures. Fluoropolymers possess outstanding chemical stability and are resistant to most solvents and corrosive chemicals. They possess excellent strength and stiffness. Excellent dielectric properties and inherently low friction behavior are also key advantages of these materials. [1]
General Engineering Plastics
Engineering thermoplastics ensure consistent mechanical properties between 5 °C and 120 °C. They can be used to replace heavier and less reliable materials, such as bronze or rubber. [2] Good chemical stability, non-toxicity, and good electrical properties are additional advantages of many engineering thermoplastics.
Polyamides (PA6, PA66, PA46)
Aliphatic polyamides, also known as nylons, are semi-crystalline thermoplastic materials. They are characterized by the amide group CONH.
Different kinds of polyamides are labeled based on the monomers used to manufacture them. The most widely used polyamides are PA6 and PA66, commonly used in packaging and textiles. They both possess good stiffness and strength at high temperatures, good wear resistance, and good impact strength at low temperatures. They are resistant to non-polar solvents and alkalis.
Due to its superior mechanical performance, PA46 is considered more suitable for engineering applications. Up to 200°C, it retains outstanding strength and stiffness. Additionally, it provides low friction combined with optimal wear resistance. Good electrical insulation and radiation resistance are additional benefits of this engineering thermoplastic. [4]
Water absorption of all polyamides is very high. Low moisture should be maintained during processing to prevent the loss of mechanical properties.
Polycarbonates (PC)
Polycarbonates (PCs) are a family of engineering thermoplastics characterized by carbonate (OCOO) bonds.
PCs possess superior impact strength and elasticity, minimal moisture absorption, excellent resistance to acids, and remarkable electrical insulation properties. This makes PC a valuable choice for demanding applications in the electronics industry. This class of thermoplastics exhibits toughness and rigidity up to 130 °C.
However, these materials are often sensitive to UV. Balancing crystallinity is crucial to maintaining optimal mechanical properties in PCs. [2]
Polyethylene terephthalate (PET)
Despite being well-known for packaging applications, Polyethylene terephthalate (PET) is also a versatile engineering thermoplastic.
Structure of PET
This thermoplastic possesses exceptional dimensional stability and wear resistance. With its low coefficient of friction, high strength, and resistance to moderately acidic solutions, it is suitable for many engineering applications. [1]
While PET is readily available and cost-effective, it has limitations, including relatively low heat resistance and susceptibility to oxidation. However, its high strength-to-weight ratio, optical properties, toughness, and recyclability make it a desirable choice in many contexts.
Polyacetals (POM-C, POM-H)
Polyacetals, also known as polyoxymethylenes (POMs), are engineering thermoplastics derived from the polymerization of formaldehyde. They are commercially available in both homopolymer (POM-H) and copolymer (POM-C) forms.
These materials offer improved dimensional stability but slightly lower wear resistance compared to polyamides. Acetals are characterized by high mechanical strength, stiffness, hardness, resilience, resistance to creep, and excellent impact strength at low temperatures. [2] They also exhibit low water absorption for outstanding dimensional stability. However, polyamides outperform acetals in impact toughness and abrasion resistance. [1]
Acetal homopolymer resins are particularly resistant to organic solvents, especially at low temperatures. POM-C is highly resistant to strong alkalis. However, polyacetals should be avoided in strong acids or oxidizing environments. Water does not significantly degrade the polymer but may cause minimal swelling (0.8%) or permeation under certain conditions.
Standard Plastics
Standard plastics are the most common thermoplastics used in consumer applications and packaging. They are inexpensive and ensure good chemical and mechanical properties below 65 °C.
Polyethylene (LDPE, HDPE, HMW-PE, UHMW-PE)
Polyethylene (PE) is the most widely used thermoplastic. It is an inexpensive material with an excellent balance of properties. In general, it offers ease of processing, toughness, and flexibility. Excellent electrical insulation across various frequencies, strong chemical resistance, and transparency make it suitable for several commodity applications.
In particular, molecular branching and molecular weight influence the characteristics of this thermoplastic. [1] As a result, there are numerous grades of PE with varying properties.
High-Density Polyethylene (HDPE) is characterized by remarkable impact resistance, tensile strength, chemical resistance, and low moisture absorption. Low-density polyethylene (LDPE) is flexible, with great impact and chemical resistance. High and Ultra-High Molecular Weight Polyethylenes (UHMW-PE) are highly versatile materials offering superb wear resistance properties, making them ideal for demanding applications like prosthetics.
Polypropylene (PP)
Polypropylene (PP) is a linear hydrocarbon thermoplastic with notable similarities to PE. PP's electrical characteristics closely resemble those of HDPE, making it an excellent insulating material, particularly for capacitor films. Its chemical resistance aligns with HDPE as well, as both polymers are highly resistant to most solvents and corrosive chemicals. [1]
Additionally, PP offers excellent resistance to stress cracking, ease of fabrication and welding, a high strength-to-weight ratio, minimal moisture absorption, and minimal porosity. These properties make PP a versatile choice for various applications, from electrical insulation to chemical-resistant components.
Download the full report for the rest of the chapter, which includes details on application of advanced thermoplastics.

References
[1] Gilbert M, editor. Brydson’s Plastics Materials. 8th ed. Oxford: Butterworth-Heinemann Elsevier; 2017.
[2] Margolis JM, editor. Engineering Plastics Handbook. 2006. New York: McGraw-Hill.
[3] Campbell RW. Down-to-earth role for imidized polymers. Machine Design [Internet]. February 7, 2002. Available from: https://www.machinedesign.com/archive/article/21813964/downtoearth-role-for-imidized-polymers
[4] Polyamide (PA) or Nylon: Complete Guide (PA6, PA66, PA11, PA12…). Omnexus [Internet]. September 27, 2023. Available from: https://omnexus.specialchem.com/selection-guide/polyamide-pa-nylon

