Integrated photonics: key enabler of bio-sensing for the fight against diseases
Point of care (PoC) testing diagnoses patients at the point of need rather than in a medical facility reducing time to diagnosis and increasing patient care.
Image: Lionix
Faster, cheaper disease diagnosis
Biosensing refers to the detection of biomolecules using an analytical device that combines a biological component with a physicochemical detector. Silicon photonic biosensors (GOOD) based on evanescent wave detection are among the leading types of technology that can deliver genuine point-of-care (PoC) devices. PoC devices will play a huge role in the global prevention of and fight against disease.
Most medical diagnostics techniques use time-consuming, expensive, and specialized techniques performed by trained technicians at the laboratory level. The distance between the patient, the diagnostic centre, and the return of results can lead to long wait times for diagnosis and expensive testing. There is a need for a portable, easy to-use, and highly sensitive lab-on-a-chip (LOC) platform for real-time diagnosis. The advantages over current methods include reduced time, costs, and a reduction in the need for highly trained staff making fast cheap testing more widely available; a scenario that has come starkly into focus during the 2020 global coronavirus pandemic.
Towards an Integrated Photonics Point of Care Future
The emerging field of Integrated Photonic (PIC) based biosensors offers advantages to the PoC industry such as miniaturization, extreme sensitivity, robustness, reliability, potential for multiplexing (multiple simultaneous tests) and mass production at low cost. This cutting-edge technology requires support and a collaborative approach to ensure its potential is maximized.
The Netherlands is a global leader in PIC research, development, and production. Various research institutes and private companies have led IP breakthroughs for more than a decade. This innovation is assisted and supported by PhotonDelta, a growth accelerator for the Dutch integrated photonics industry. To further innovation in the area of IP biosensing, PhotonDelta has created a roadmap for the technology (EXTERNAL) that supports the mission of PhotonDelta to grow the business based on integrated photonics in the Netherlands. The roadmap illustrates the path towards medical devices based on integrated photonics.
This article will outline the role of integrated photonics in enabling the development of biosensors that will be a key part of our future health care systems while highlighting the core points from the PhotonDelta roadmap, including:
- Describing the current trends and drivers.
- Outlining the technological solutions based on integrated photonics.
- Providing an overview of the challenges and opportunities.
- Outlining the industry ecosystem.
Point of Care testing
The Point-of-Care testing market is significant and growing quickly, even in pre-Covid times. The need for advanced point of care testing has become increasingly clear during the coronavirus pandemic. The value growth of the PoC market is expected to grow from $4.8B in 2019 to $10.1B in 2025, at a CAGR of 13.0%. The associated biosensor device market (microfluidic chips) was valued at $500M in 2019 with an estimated growth to $777M by 2025 at CAGR of 7.7%.1
The drivers for an increase in PoC testing include the severe cost pressure on global health care systems as a result of the aging population and a focus to reduce the impact of the world's deadliest diseases.
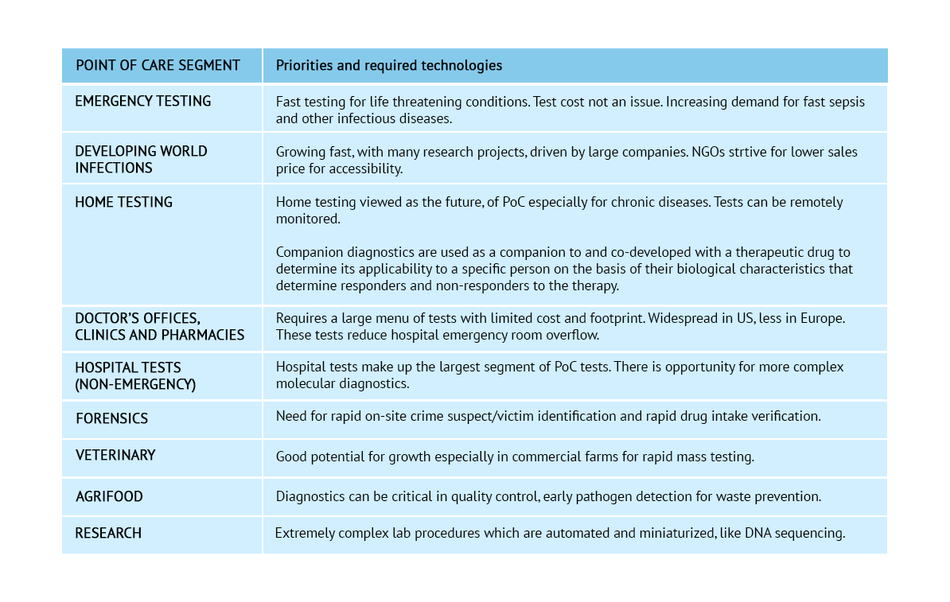
Point of Care testing exists in a wide range of segments. Each has its own set of priorities and required technologies.
 Timeline and trends
Timeline and trends

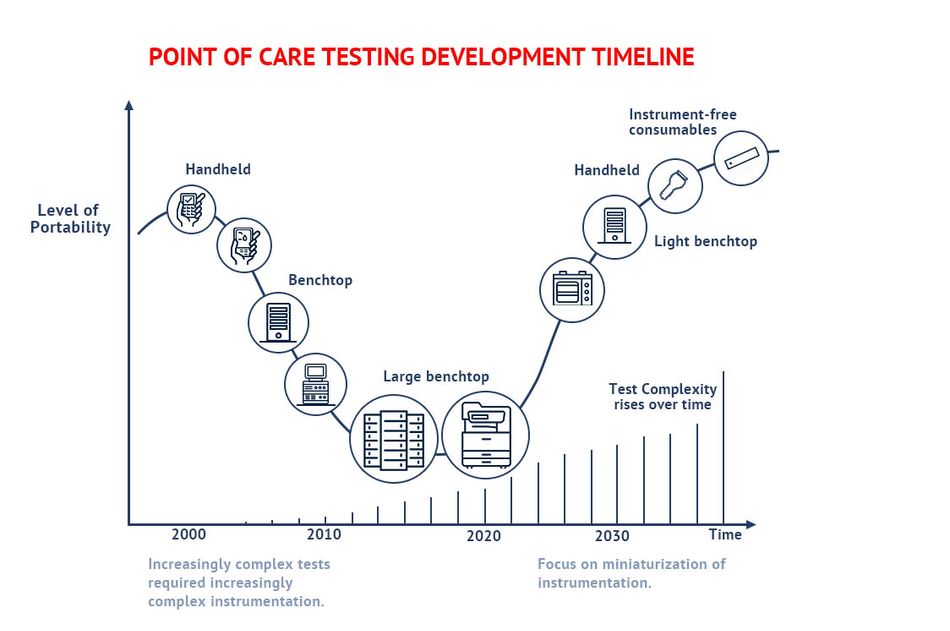
PoC testing has undergone a significant transformation over the last two decades. In the early 2000s, PoC testing enjoyed a huge breakthrough with the wide availability of simple tests such as blood glucose levels. These handheld devices offered fast results, although their cost was still relatively inhibitive for mass availability. In the resulting years, increasingly complex tests required increasingly complex instrumentation. Large, expensive, and lab-bound testing instruments were common. These instruments decreased in size as breakthroughs in Integrated Circuits (IC) and other technologies allowed, PoC testing demands a further reduction in the size of instruments, with the long-term goal to deliver instrument-free consumables.
Integrated photonics biosensor technology
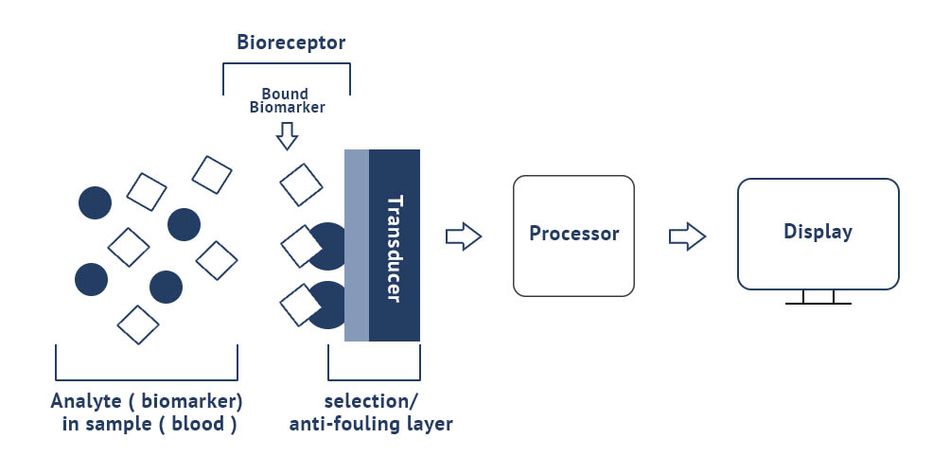
Integrated photonics biosensor technology has the potential to deliver fast, affordable, and highly accurate biosensor testing applications useful for PoC. Integrated biosensors are miniaturized in Integrated Circuit-like processes and can be produced in large quantities which leads to very low cost and thus disposability. Because of their very small size and the integration processes, a limited sample volume is needed, the time to result is fast, and the sensor area can be filled with many sensing structures for multiplexing.
The integrated biosensor concept hinges on the biomarker/bioreceptor interaction. The integrated biosensor device combines a biological or biomimetic recognition element with a transducer which converts a specific (bio)chemical interaction into a measurable signal. The selective biological receptor determines the specificity and response time of the test in combination with the layer of the sensor which determines where the receptors are attached while being antifouling elsewhere. The main bioreceptors are antibodies, DNA strands, aptamers or enzymes, and biomimetic materials. The method of inclusion or immobilization of the receptor on the transducer surface is critical for performance. The more different types of bioreceptors that can be defined on the active surface, the more powerful the biosensor is.
SiN offers advantages
PIC biosensors using Silicon Nitride (SiN) waveguides offer several intrinsic advantageous properties. They work in a very wide wavelength range from visible to near-infrared, avoiding the water absorption window of water and allowing fluorescence detection. This wavelength range makes it easy to combine them with a cheap laser source, reducing their cost and size. Biosensors based on SiN PICs are highly sensitive. A very low detection limit can be achieved by using self-referencing optical structures that eliminate sources of noise like temperature variations. For more technical detail on PIC-based biosensors, read our other article here. (BAD)
Cost and size
PIC biosensor technology can deliver the low cost, portable tests that the healthcare industry demands. In general, cost per test must be lower than clinical lab testing, unless the value provided by the short time to result is very high, then higher cost per test is worthwhile. The main route to driving down the cost is mass manufacturing the same way the IC industry has done for ICs: the production of many million PICs per year based coming from large substrate wafers which are processed in highly automated lines.
The drive is now towards instrument-free consumables. This means shrinking the analyzer and test chip to a disposable which can easily be held in hand. The coronavirus ‘fast tests’ are a good example of this.
Multiplexing and time to result
One of the key advantages PIC based PoC testing offers is the ability for significant multiplexing. These tests are able to interrogate several targets at one time resulting in more accurate and streamlined testing procedures. The trend for tests with only 2 or 3 bioreceptor types are ‘fast tests’ with results delivered in under 15 minutes. Tests with significant multiplexing, for so-called syndromic testing, such as microbiological screening or cancer profiling that may have more than 200-plex should aim to be returned in under an hour or at least significantly faster than conventional tests.
Challenges and Opportunities
An important long-term goal for PIC biosensor PoC testing is to remove the need for any instrument (benchtop or handheld) instead have disposable testing instruments using smartphones for display purposes. The key targets of reducing cost, addressing appropriate sensitivity, managing time to results, and increasing multiplexing drives the field forward, however, each of these targets will be hit at different times depending on their own set of technological challenges. Given the complexity of the market, profitable applications can be envisioned to arise already when only one or a few of these targets are met.
Opportunities
Outside of efficient and low-cost PoC testing, there are several drivers in life sciences and health care that can be addressed by integrated photonics. These include the continuous monitoring of biomarkers (wearable, minimally invasive, ingestible, or implantable). There is also the possibility of a combination of monitoring of biomarkers with simultaneous therapy, within the framework of personalized medicine, such as in-situ diagnostics of organ-on-a-chip systems.
Challenges
Challenges for the development of PIC-based PoC tests centers on a trade-off between cost and robustness/reliability. There are several directions the technology could take depending on the timing of the maturation of some of the technology and the focus that leading developers take. A multi-disciplinary approach towards assay development, biochemistry, surface functionalization, microfluidics, and an integrated approach to industrialization is necessary for high cadence innovation.
Industry ecosystem
The biosensing ecosystem is complex and requires significant collaboration between diverse groups to ensure continued increased innovation. A high-level overview of the industry's leading players and influencers demonstrates how a collaborative approach to PIC biosensors for PoC is essential.
Platform provider: Offers a hardware platform (instrument & disposable) that enables the integration of standardized assays, with sample preparation and detection.
Assay provider: Comes with its own assay (reagents) and collaborates with the platform provider to integrate the assay on the hardware.
Disposable manufacturer: Combines the elements from the platform provider and the assay provider. May also obtain optical biosensors from the foundry.
Distributor: Is the conduit to the end-user. May also be the platform provider and even the assay provider.
End-user: Any customer having purchased the instrument from the platform provider
Others: providing packaging, assembly, and testing equipment; R&D services.
Next steps
Even pre-Covid, there is a sizeable, fast-growing market for PoC testing, with a large number of potentially profitable product/market segment combinations. The most significant growth area is in complex molecular diagnostics that require low-cost disposable biosensors that deliver fast results and have extensive multiplexing capability. A collaborative approach, particularly between diagnostic companies and platform providers is key for globally relevant innovation.
Download the PhotonDelta roadmap here. (EXTERNAL)
About the sponsor: PhotonDelta
PhotonDelta (GOOD) is a growth accelerator for the Dutch integrated photonics industry. Its objective is to promote the adoption of integrated photonics and support companies to get prioritized access to the Dutch Integrated Photonics supply chain. Furthermore, PhotonDelta runs a new business creation program that provides companies, ranging from startups to SMEs and big organizations, with access to strategy, market, and funds.
Through its wide network within the industry, PhotonDelta has gathered insights on market trends, challenges, and product roadmaps. This information is used to drive business creation, investments, and strategic technology roadmaps. Furthermore, PhotonDelta actively supports start-ups and SMEs to facilitate their involvement with the Dutch integrated photonic supply chain. This includes facilitating options for establishing a solid presence in the Netherlands.

References and further reading
1. Point-of-Need 2020 – including PCR-Based Testing- report-www.yole.fr