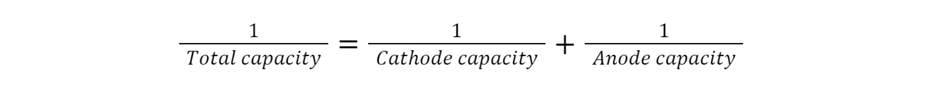Surface engineered nickel-rich cathode material enabling high power discharge for Li-ion battery applications
This study presents a simple approach to preparing a robust surface modified nickel-rich cathode material for Li-ion battery application. A detailed comparison of the importance of cathode and the present study to make a big difference in achieving the low-cost future battery are explained.
This article is a part of our University Technology Exposure Program. The program aims to recognize and reward innovation from engineering students and researchers across the globe.
Community voting is now live. Vote for your favorite submission by visiting: University Technology Exposure Program Community Vote.
Overview of the technology
The popularity of electric vehicles (EVs) demands high-performance batteries to achieve its long-distance and high-power objectives. To satisfy these demands, nickel-rich LiNixMnyCozO2 (NMC, x+y+z=1; where, x≥0.5) materials are promising cathode materials due to their high specific capacity. However, during the charge-discharge of these materials, especially at higher voltages (> 4.2 V vs. Li+/Li) [1], it undergoes side reactions leading to internal structure collapse and drastic capacity loss. Surface coating to the NMC particle is proven in the literature to overcome this problem. However, considering the surface coating of the particle with low thickness 50 < nanometer and homogeneous coating, coating the nanometer size particle are easier than micron-sized NMC. The huge size of the particle often leads to inhomogeneity. To overcome this problem in this study we have coated NMC particles with an amorphous carbon coating, which is easy and has control over its thickness. However, the literature also reported that the carbon coating to NMC material in an inert atmosphere changes its internal structure and leads to a non-favorable battery material. So, carefully in this study, these problems are solved.
What sub-systems is the technology composed of?
The present study explains the surface engineer of NMC particle, an approach of polymerizing furfuryl alcohol monomer on the surface of NMC particle, followed by a calcination step is carried out to achieve a homogenous carbon coating.
What tasks do they fulfill?
The surface engineered NMC particles are able to perform at a 10C discharge rate (C rate means the current applied and it is 1C= 160 mA g-1).
How are these sub-systems integrated?
These surface-coated particles can be used as the active material for cathode electrode preparation (electrode comprises of active material (here it is NMC), conducting carbon and polymeric binder) without further modification to the conventional electrode processing.
Issues/obstacles resolved through clever component reengineering or innovative solutions
As discussed in the previous section, nanometric level surface coating on the micron-sized particle is difficult. Along with carbon coating on the NMC particle is difficult because of it is metal oxide nature. Usually, the metal oxide structures oxygen reacts with carbon and collapses the original structure during the carbon coating to NMC particles. However, in the present study, both the issues are carefully solved by choosing a suitable carbon coating procedure.
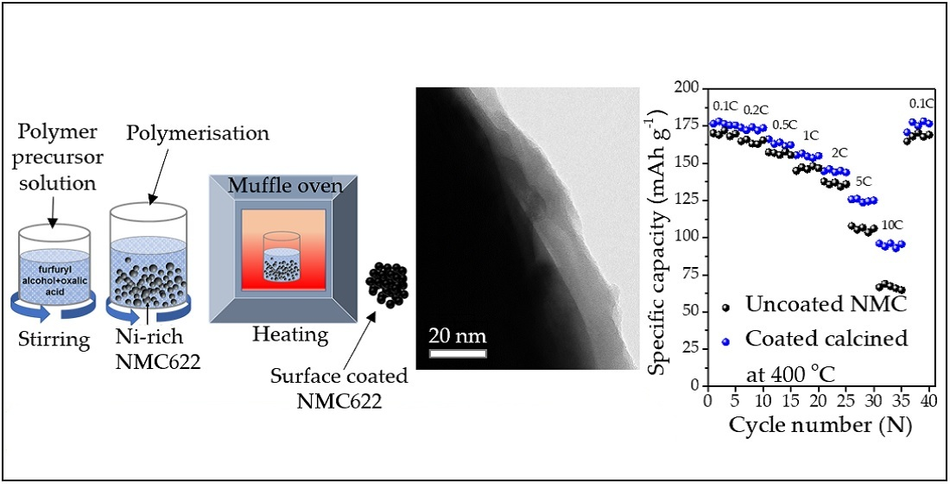
Additionally, the surface coating process to the NMC should be easy and cost-effective. In the present study, the coating procedure does not involve a sophisticated setup and expensive raw materials; hence it is cheaper and easily scalable. Figure 1 shows the schematic illustration of the surface coating procedure of NMC particles and its confirmation by transmission electron micrography technique, which shows ~15-nanometer thick homogeneous coating. Further, the different current load (0.1C to 10C) tests shown in Figure 1 describe the coated materials performance improvement at higher current loads. Especially at the 10C current rate, the coated NMC particles deliver ~50% capacity improvement. The 10C rate means discharging the cell at 6 minutes (1C = 60 minutes of operation).
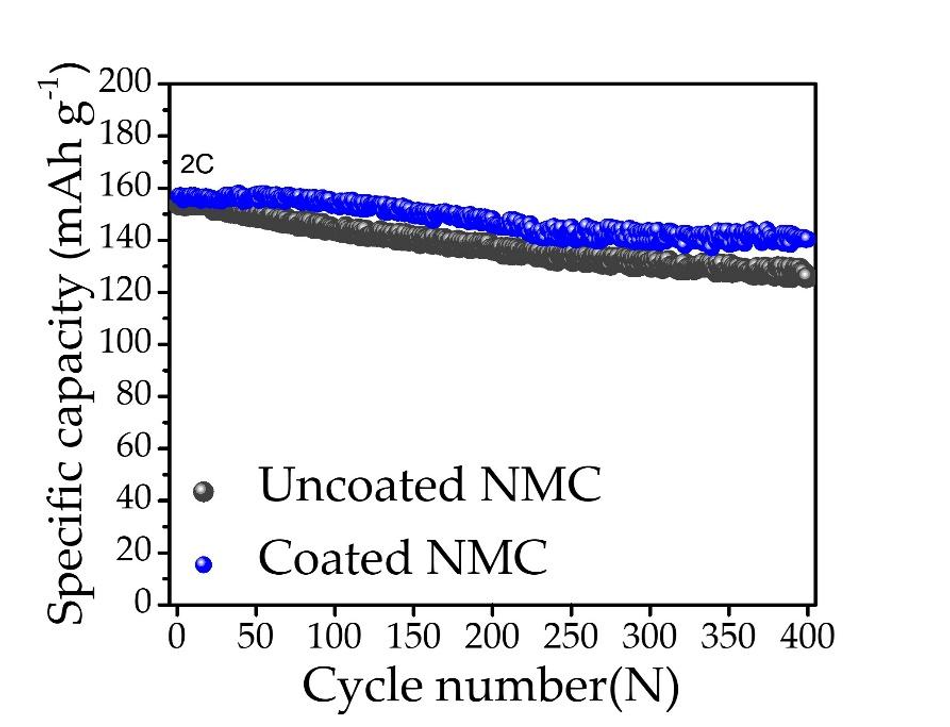
The present study shows the practical application of the surface-coated NMC particle in Li-ion cells, which can be used in other kinds of Li-ion cells such as solid-state batteries. The enhanced performance of the coated material will substantially improve the high current and high energy performances of Li-ion batteries.
Different applications of the technology
The surface-coated NMC particle improves the Li-ion batteries total available capacity and high-power performance. Which can be successfully used in applications such as:
Electric vehicle,
Drone application,
Military tool kits,
Power tools,
Grid storage,
Consumer electronics and many more.
How does it affect the industry and/or society?
Due to the low carbon emission during electric vehicle operation, they are getting popularized. However, they need to be low cost, higher life, and high capacity for their success. Battery materials play an essential role in achieving these demands. Nearly 60% of the battery cost comes from the battery materials (cathode, anode, electrolyte, separator, other material, and labor cost). Interestingly 50% of the total battery material cost comes from the cathode material. Hence it needs to be cheaper and more effective [3]. The cathode treatment or modification process should be more reasonable, not adding up extra handling costs.
Additionally, If we consider both the anode and cathode are connected in series and the total capacity of the battery is the sum of individual cathode and anode capacities as given by Equation 1
For a fixed cathode (here it is NMC), only increasing the anode capacity will not improve the total capacity of the overall cell. Hence to enhance the capacity, the cathode capacity needs to be improved. And in todays scenario, many high-capacity anodes such as silicon and tin-sulfide are available [4,5]. High-capacity cathodes are essential to match the high capacity of these anodes. In this way, nickel-rich cathodes are a suitable choice. The present study of surface coating improves the stability of these high-capacity cathodes at a higher current rate, which is also one of the key requirements for electric vehicle applications [6].
Technical Specifications
The present technology is a simple and easily scalable approach. The detailed procedure of the method is available in the article [2], which uses the polymerization of furfuryl alcohol monomer on the surface of NMC particle followed by a calcination process at 400 oC, which induces the carbon coating thickness of ~15 nanometer on the surface of NMC particles.
References
[1] A. Manthiram, B. Song, W. Li, A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries, Energy Storage Mater. 6 (2017) 125.
[2] A.R. Kathribail, A. Rezqita, D. Lager, R. Hamid, Y. Surace, M. Berecibar, J. Van Mierlo, A. Hubin, M. Jahn, J. Kahr, High-Performance Amorphous Carbon Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries, Batter. . 7 (2021). https://doi.org/10.3390/batteries7040069.
[3] No Title, (n.d.). https://qnovo.com/82-the-cost-components-of-a-battery/.
[4] D. Larcher, S. Beattie, M. Morcrette, K. Edström, J.-C. Jumas, J.-M. Tarascon, Recent findings and prospects in the field of pure metals as negative electrodes for Li-ion batteries, J. Mater. Chem. 17 (2007) 3759–3772. https://doi.org/10.1039/B705421C.
[5] N. Nitta, F. Wu, J.T. Lee, G. Yushin, Li-ion battery materials: present and future, Mater. Today. 18 (2015) 252–264. https://doi.org/https://doi.org/10.1016/j.mattod.2014.10.040.
[6] M. Weiss, R. Ruess, J. Kasnatscheew, Y. Levartovsky, N.R. Levy, P. Minnmann, L. Stolz, T. Waldmann, M. Wohlfahrt-Mehrens, D. Aurbach, M. Winter, Y. Ein-Eli, J. Janek, Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects, Adv. Energy Mater. 11 (2021) 2101126. https://doi.org/https://doi.org/10.1002/aenm.202101126.
About the University Technology Exposure Program 2022
Wevolver, in partnership with Mouser Electronics and Ansys, is excited to announce the launch of the University Technology Exposure Program 2022. The program aims to recognize and reward innovation from engineering students and researchers across the globe. Learn more about the program here.