Miniaturization of Components: Enabling Next-Generation Medical Devices and Implantables
Discover how component miniaturization powers next-generation medical devices and implantables, improving portability, reliability, and lifespan.
Introduction
Electronic components are becoming increasingly compact, significantly altering the way engineers design modern systems. This ongoing trend, known as miniaturization, involves developing smaller capacitors, inductors, sensors, and integrated circuits, all while maintaining, or even enhancing, their performance. Many of these downsized components can deliver superior efficiency, precision, and reliability compared to their larger predecessors.
In medical technology, miniaturization holds particular significance. Devices inserted into the body or used for monitoring must be small, lightweight, and energy-efficient. However, they also need to meet strict safety and reliability standards, as they operate in some of the most demanding environments – the human body. Every millimeter saved and every nanowatt conserved leads to longer battery life, smaller devices, and better outcomes for patients.
In this article, we’ll look at how smaller components are pushing the boundaries of what’s possible in medical technology. We’ll also demonstrate how Murata is helping lead this shift with purpose-built components that strike a balance between size, power consumption, and performance.
 Miniaturized Electronic Components; Image Credit: PartstatEngineering Imperatives for Component Selection
Miniaturized Electronic Components; Image Credit: PartstatEngineering Imperatives for Component Selection

In life-critical medical systems, component integrity is key. Unlike consumer electronics, such systems operate with very little tolerance for error. The failure or performance drift of a single capacitor, inductor, or sensor can jeopardize device functionality and impact patient safety.
Reliability and stability at the component level are, therefore, not merely design considerations; they are essential for regulatory compliance and clinical trust. That’s why only medical-grade components, specifically designed for their intended use, should be considered. Engineers must look beyond the basic specifications of components and ensure that the parts in their designs are stable over time, safe for use, can withstand heat, and continue to function correctly under constant use.
Selecting the wrong components or those that are not specifically designed for the application can result in poor battery performance, low signal quality, or even complete device failure.
Further Reading: Miniaturization Meets Performance: Designing Reliable, Wearable Devices for Diabetes Care
Technical Challenges in Miniaturization of Components
Making components significantly smaller while maintaining their reliability presents real engineering challenges. The common issues relate to their mechanical design, electrical performance, and compliance with strict medical regulations.
The first issue is mechanical. As parts shrink, they still need to handle vibration, pressure, and repeated handling during manufacturing. Thinner materials are more likely to crack or wear out, so the choice of materials and their packaging is critical.
Electrically, things become more challenging! Smaller components must use less power while maintaining stable signals. It is also much harder to control leakage current, parasitic effects, and reduced capacitance or inductance when components are positioned closely together. These issues can disrupt the signal and impact the overall device's performance.
Electromagnetic interference (EMI) is another issue. Due to less space, signals can interfere with each other through crosstalk or coupling. To avoid this, innovative layout strategies and reliable EMI suppression are needed.
Environmental conditions must also be considered. Medical and implantable devices must withstand ongoing shifts in temperature, humidity, and long-term exposure to bodily fluids.
The manufacturing process is also challenged by miniaturization. Even tiny shifts in the process can affect how a part performs. This requires sophisticated tools, precise fabrication, and thorough testing and inspection to maintain the high quality of the components.
Finally, all of these challenges must pass the tight deadlines set by medical safety regulations such as ISO 13485 and IEC 60601.
How Specialized Miniaturized Components Drive Innovation
Miniaturized components are not only about saving space. They directly shape what is possible in modern medical and implantable devices. The advantages of reducing component size include:
Space Efficiency: The first and most crucial benefit is space efficiency. Smaller components take up less space and free up board area, allowing engineers to add other elements, such as sensors, wireless connectivity modules, and advanced power management.
Closer-to-Target Placement: Smaller parts make it easier to place electronics closer to where they’re needed. In medical implants, this means devices can sit right at the treatment site, such as inside the heart for a leadless pacemaker or near a nerve for a neurostimulator.
Functional Integration: Functional Integration is another big advantage. When multiple functions, such as passives, sensors, and RF, are combined into a single unit, the number of solder joints and connections is reduced. This ultimately reduces the number of failure points.
Power Efficiency: When components are placed close together, the electrical connections between them are shorter and simpler, resulting in improved power efficiency. This helps reduce unwanted effects such as stray resistance or small energy leaks. As a result, less power is wasted, which makes the device more efficient. In medical implants, this means smaller batteries can be used, and they don’t need to be replaced as often, which is a big win for both patients and doctors.
New Form Factors: Innovative designs become possible when electronics shrink. Minimally invasive devices such as injectable sensors or flexible implants that conform to body structures rely on miniaturized, integrated components to achieve their design goals.
Durability: The devices, which include miniaturized components, also last longer. Using fewer separate parts and combining functions into one unit makes them more durable. This helps prevent failures from stress or worn-out connections.
The impact of these engineering benefits can be seen in advanced medical devices such as leadless pacemakers, implantable neurostimulators, cochlear implants, bionic eyes and hands, pain management devices, spine stimulators, implantable glucose management and bladder stimulators, as well as many other cutting-edge medical aids.
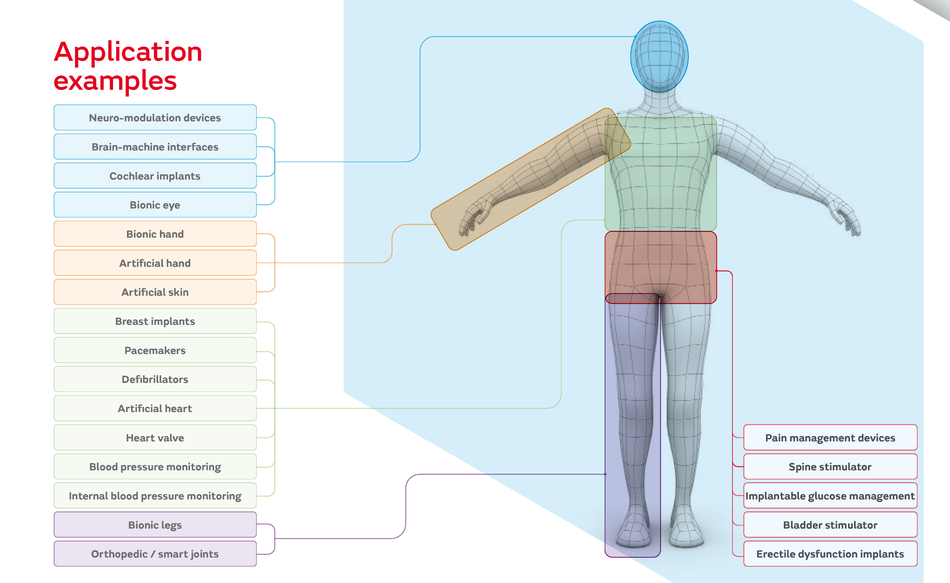 Application Examples; Image Credit: Murata How Murata Addresses Miniaturization Challenges
Application Examples; Image Credit: Murata How Murata Addresses Miniaturization Challenges

Murata provides purpose-built components to meet the strict requirements of implantable medical devices, combining ultra-small size, long-term reliability, low power use, and full compliance with medical standards.
Multi-Layer Ceramic Capacitors (MLCCs)
Murata offers one of the industry’s broadest ranges of Multi-Layer Ceramic Capacitors (MLCCs). These medical-grade MLCCs are used in Class D implantable devices, such as for cardiac rhythm management (defibrillators, pacemakers, or insertable cardiac monitors) and neurostimulation/neuromodulation therapies (e.g., SCS, SNS, VNS, DBS, etc.).
These capacitors provide stable characteristics over a wide range of voltages, ensuring dependable performance in long-life medical applications.
Silicon Capacitors and Integrated Passive Devices (IPDs)
Alongside MLCCs, Murata also provides Medical Grade Silicon Capacitors (MGSCs) and design-specific Integrated Passive Devices (IPDs).
MGSCs are highly reliable and have an extremely low profile, down to 100μm. These capacitors are also highly stable over voltage and temperature ranges.
Murata also provides design-specific IPDs. These custom IPDs reduce component count by embedding multiple passives into a single package. This can be a capacitor array or a complex IPD embedding different types of passives and connections. This approach enhances system integration and saves valuable board space.
In addition, stackable IPDs allow designers to place capacitors closer to the active device, which minimizes parasitic effects, improves signal integrity, and enhances overall reliability.
Noise and EMI Suppression Components
For noise and EMI suppression in smaller circuits, Murata offers Power Inductors for Bluetooth® low energy applications and compact RF inductors for medical applications.
The LQM18PH_FC series from the power inductor family provides low RDC with high current capability, which makes it suitable for power line filtering in space-constrained circuits.
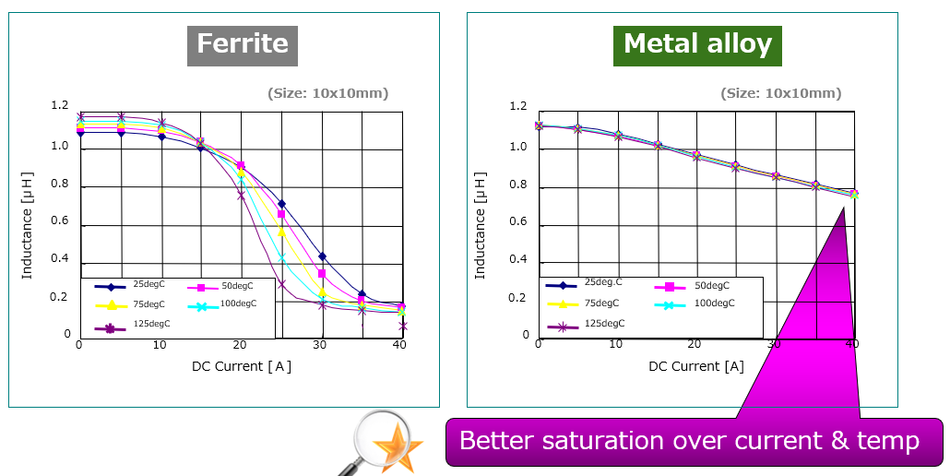
For high-frequency applications, the company provides the DFE2MCPH series, which is a lineup of metal alloy power inductors that delivers superior performance compared to traditional ferrite-based options. Although it comes in a slightly larger 2016M (0806 inch) case size, the enhanced performance characteristics have made it especially appealing to medical customers seeking higher reliability and efficiency.
Murata also offers the LQW18CN_0H series, a wire-wound RF inductor available in a wide inductance range from 4.9 nH to 650 nH. These components are available in 1608M (0603 inch) case size — the same footprint as the LQM18PH_FC mentioned earlier.
MEMS Sensors
Murata produces bare MEMS sensor elements for 3-axis acceleration (SCG30E series) and absolute pressure (SCB10H series), based on capacitive 3D-MEMS technology.
MEMS accelerometer sensing elements are hermetically sealed, have high Capacitance sensitivity (>150fF/g), high isolation resistance (>10GΩ), and low parasitic capacitance. These sensor elements are very small in size (2.0mm x 2.0mm x 0.95mm) and are highly stable and accurate over temperature.
MEMS pressure sensor elements (SCB10H series) are compact sensor elements (1.4 × 1.4 × 0.85 mm). These elements can withstand shock pressures over 200 bar and have high insulation resistance above 10 GΩ. They deliver high sensitivity with a 4 pF capacitance change and are offered in pressure ranges from 1.2 to 25 bar.
NTC Thermistors
NTC thermistors by Murata provide precise body temperature monitoring and compensation. They are available in compact sizes from 01005 to 0603, offering tight resistance tolerances (as low as ±0.5%) and high reliability.
Connectivity Modules
Murata also offers a wide variety of ultra-small connectivity modules (Bluetooth® Low Energy, Wi-Fi™, LPWA, LoRa, and UWB). These modules integrate antennas and are regulatory certified. Their compact footprint makes them ideal for secure telemetry in implants and medical wearables.
Conclusion
Miniaturization is more than a design trend; it is fundamental to the future of medical and implantable devices. Every size reduction must be matched by uncompromising reliability, efficiency, and compliance, making component selection a critical engineering decision.
This progress depends on collaboration between OEMs, engineers, and component specialists, such as Murata, as their miniaturized technologies enable the next generation of reliable and safe medical devices.
Visit Murata’s website to learn more about their miniaturization solutions for medical technology.